Austin, Mark, Egan, Oliver James, Tully, Ray and Pratt, Albert C. (2007) Quinoline synthesis: scope and regiochemistry of photocyclisation of substituted benzylidenecyclopentanone O-alkyl and O-acetyloximes. Organic and Biomolecular Chemistry, 5 (23). pp. 3778-3786. ISSN 1477-0520
Abstract
Irradiation of substituted 2-benzylidenecyclopentanone O-alkyl and O-acetyloximes in methanol provides a convenient synthesis of alkyl, alkoxy, hydroxy, acetoxy, amino, dimethylamino and benzo substituted annulated quinolines. para-Substituents yield 6-substituted-2,3-dihydro-1H-cyclopenta[b]quinolines with 8-substituted products being obtained from ortho-substituted starting materials. Reactions of meta-substituted precursors are highly regioselective, with alkyl substituents leading to 5-substituted 2,3-dihydro-1H-cyclopenta[b]quinolines and more strongly electron-donating substituents generally resulting in 7-substituted products. 2-Furylmethylene and 2-thienylmethylene analogues yield annulated furo- and thieno-[2,3e]pyridines respectively. Sequential E- to Z-benzylidene group isomerisation and six [pi]-electron cyclisation steps result in formation of a short-lived dihydroquinoline intermediate which spontaneously aromatises by elimination of an alcohol or acetic acid. For 2-benzylidenecyclopentanone O-allyloxime, singlet excited states are involved in both steps.
Metadata
| Item Type: | Article (Published) |
|---|---|
| Refereed: | Yes |
| Subjects: | Physical Sciences > Organic chemistry Physical Sciences > Chemistry |
| DCU Faculties and Centres: | DCU Faculties and Schools > Faculty of Science and Health > School of Chemical Sciences |
| Publisher: | Royal Society of Chemistry |
| Official URL: | http://dx.doi.org/10.1039/b711620a |
| Use License: | This item is licensed under a Creative Commons Attribution-NonCommercial-Share Alike 3.0 License. View License |
| ID Code: | 119 |
| Deposited On: | 14 Jan 2008 by DORAS Administrator . Last Modified 19 Jul 2018 14:40 |
Documents
Full text available as:
Preview |
PDF
- Requires a PDF viewer such as GSview, Xpdf or Adobe Acrobat Reader
144kB |
Preview |
PDF
- Requires a PDF viewer such as GSview, Xpdf or Adobe Acrobat Reader
744kB |
![[thumbnail of abstract_image.gif]](https://doras.dcu.ie/119/3.hassmallThumbnailVersion/abstract_image.gif) 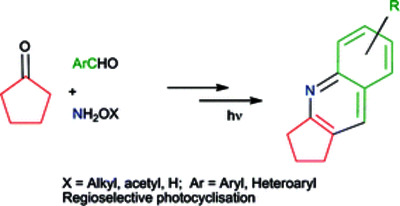 Preview |
Image (GIF)
6kB |
Metrics
Altmetric Badge
Dimensions Badge
Downloads
Downloads
Downloads per month over past year
Archive Staff Only: edit this record